We are trying to make the best product in the world.
Sign of pressure
The definition of pressure is the force acting on the unit area, in which the magnitude of the force (Kgf) acting per 1 Cm2 is most commonly used, i.e. Kgf/Cm2. Pressure may also be expressed in terms of the corresponding height of the mercury (mmHg) and the height of the water column (mmHg).
Recently, the spread of the International Unit System (SI Unit System) is expanding for the purpose of international unit unification, in which the force acting per square meter, N/m2, the size of N (Newton), is expressed as Pa (Pascal).
In this case, Pa is a fairly small unit and often uses either Kpa (kilo-pascal) or Mpa (mega-pascal) that is 1,000 times larger.
Bar(bar) is used as the atmospheric pressure unit.
Comparison of unit
| / | kgf/Cm2 | bar | PSI | kPa |
|---|---|---|---|---|
| kgf/Cm2 | 1 | 0.9807 | 14.2233 | 98.07 |
| bar | 1.0197 | 1 | 14.5037 | 100 |
| PSI | 0.0703 | 0.06894 | 1 | 6.8747 |
| kPa | 0.01019 | 0.01 | 0.14503 | 1 |
Standard of pressure
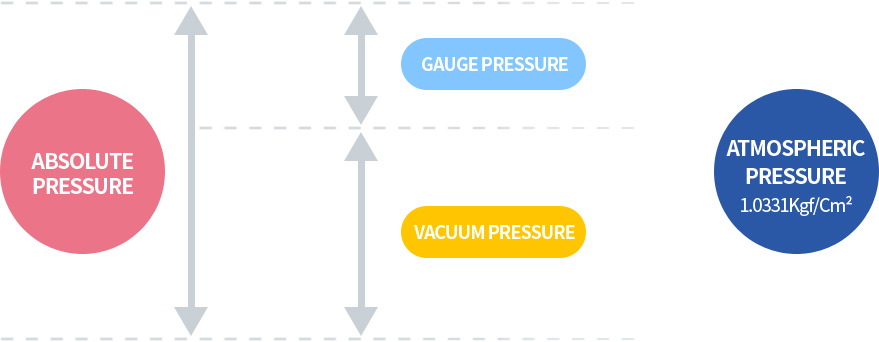
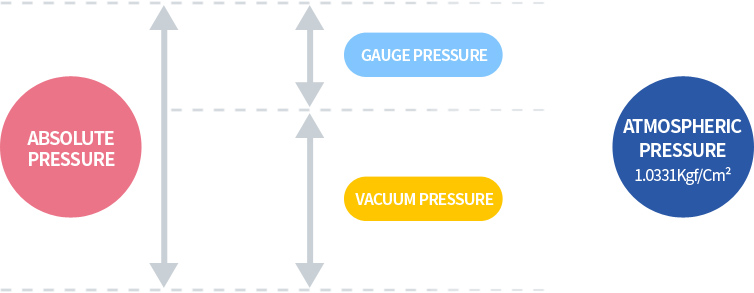
The relationship of pressure temperature volume of air
P = Absolute pressure of air [Kgf/Cm2abs]
V = Specific volume [m3/kgf]
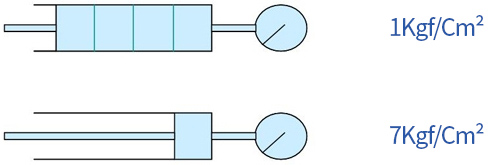
For example, if air pressure of 1Kgf/Cm2G (≒2Kgf/Cm2abs) is compressed to a volume of 1/4 as shown, the pressure will be 7Kgf/Cm2G (≒8Kgf/Cm2abs), increasing by approximately 4 times the absolute pressure.
This relationship is called BOYLE's law.
본 사이트는
Internet Explorer 8 이하 버전을
지원하지 않습니다.
Internet Explorer 9 이상으로 업데이트 하거나
크롬, 파이어폭스, 오페라, 사파리 최신 브라우저를 이용해 주십시오.
불편을 드려 죄송합니다.